CryoLetters Volume 47 - Issue 2
CryoLetters 47 (2), 72-89 (2026)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr26210110112
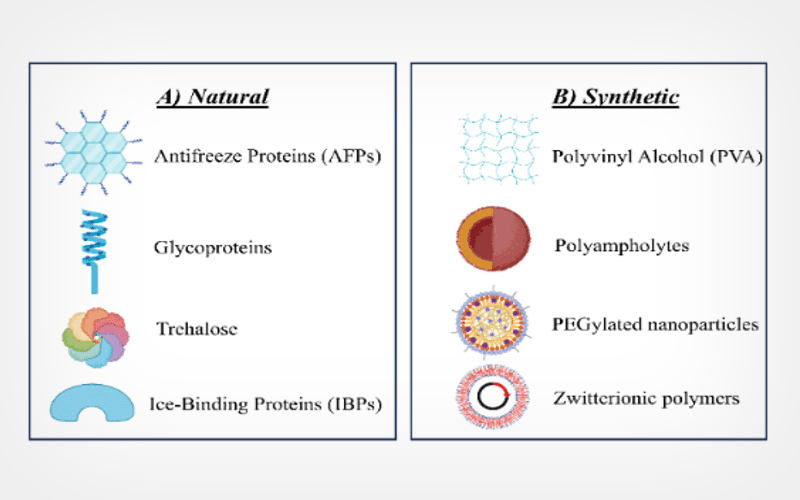
PERSPECTIVE: Nanoengineered cryoprotectants and smart biomaterials for precision sperm cryopreservation: a new frontier in male fertility preservation
A. S. Vickram*, S. Bharath, B. Bhavani Sowndharya, C. Prasanth,
D. Jenila Rani and A. Saravanan
- Department of Biotechnology, Saveetha School of Engineering, SIMATS, Chennai, 602105, India.
*Corresponding author’s E-mail: vickramas.sse@saveetha.com
Abstract
Sperm cryopreservation is a key technology in reproductive medicine, providing patients the possibility to retain viability before medical interventions or age-related decline. Despite its clinical significance, current cryopreservation procedures suffer substantial limits due to cryoinjury, most notably from intracellular ice formation, osmotic imbalance, membrane instability, and oxidative damage. These conditions significantly affect sperm motility, viability, and genetic integrity post-thaw. To overcome these problems, recent breakthroughs have focused on merging nanotechnology and smart biomaterial science to produce next generation cryoprotectants and preservation systems. Nanoengineered cryoprotectants comprising customized nanomaterials such as liposomes, polymeric nanoparticles, and biologically derived exosomes have shown improved membrane protection, effective antioxidant delivery, and reduction of ice nucleation compared to traditional agents. Early preclinical tests reveal that these alterations considerably enhance post-thaw sperm sustainability, minimize DNA fragmentation, and sustain functional ability for fertilization. Moreover, the combination of individualized cryopreservation protocols leveraging microfluidic technology and embedded biosensors allows unprecedented control and real-time monitoring of cryopreservation quality suited to unique patient demands. Despite these gains, further study into nanotoxicity, long-term safety, and regulatory standards is necessary before widespread clinical adoption. Collectively, nanoengineered cryoprotectants and smart biomaterials constitute a promising new frontier, seeking to enhance male fertility preservation with higher efficiency, safety, and tailored solutions.
Keywords: nanoengineered cryoprotectants; polymeric nanoparticles; reproductive health; smart biomaterials; sperm cryopreservation; trehalose
CryoLetters 47 (2), 90-95 (2026)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr26210110312
The protective effects of PHD-inhibitor GSK360A against ischemia injury and improve the function of frozen-thawed rat testicular graft
Jian-Min Zhang*, Yong Qi and Jie Sun
- Weifang Nursing Vocational College, No. 9966, Yunmenshan South Road, Qingzhou City, 262500, China.
*Corresponding author’s E-mail: jmzwfhl@163.com
Abstract
Background
Spermatogonia is sensitive to the toxicity of chemotherapy and/or radiotherapy agents. Cryopreservation of testis tissue may offer fertility restoration for pre-pubertal male cancer survivors.
Objective
To investigate the effects of PHD-inhibitor GSK360A on the function of testis graft following cryopreservation. Besides, the underlying mechanism is explored.
Materials and methods
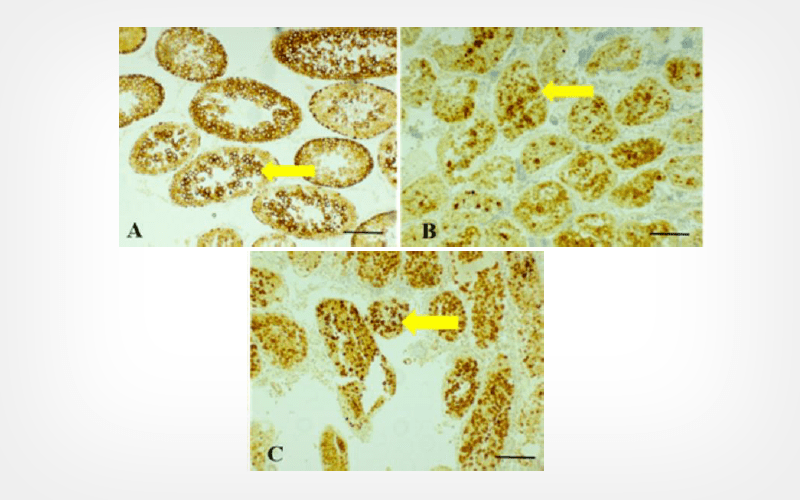
Wister rats were randomly divided into three groups: fresh control group (neither freezing nor autograft), vehicle group (freezing + autograft + vehicle), and GSK360A group (freezing + autograft + GSK360A). The rate of apoptotic Leydig cells and expression of RIPK1 and caspase-8 were assessed on day 7 post-transplantation. At the 30th day post-transplantation, the number of spermatogonia, as well as microvessel density of graft were measured.
Results
GSK360A statistically increased the number of spermatogonia per round tubule, redued the rate of apoptotic Leydig cells, and enhanced the microvessel density of the graft. Furthermore, diminished protein expression of RIPK1 and enhanced caspase-8 expression were found in the GSK360A group than in vehicle group.
Conclusion
GSK360A exhibits protective effects on testicular frozen graft by increasing the number of spermatogonia and reducing the rate of apoptotic Leydig cells. Its mechanism may be related to attenuating ischaemic injury and switching cellular death mode from necroptosis to apoptosis in testis grafts.
Keywords: cryopreservation; GSK360A; PHD-inhibitor; testicular tissue; transplantation
CryoLetters 47 (2), 96-107 (2026)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr26210110612
Effects of procedure manipulation and induced nucleation on sperm cryopreservation
Weijie Li1, Ning Wu1, Qin Qi2, Zhixin Li1,3,4 , Baolin Liu1,3,4*, Yunfeng Xue5,
Jianguo Qu5 and Yunxin Cao5
- Institute of Biothermal Science and Technology, University of Shanghai for Science and Technology, Shanghai 200093, China.
- Department of Publishing and Dissemination, Shanghai Publishing and Printing College, Shanghai 200093, China.
- Shanghai Technical Service Platform for Cryopreservation of Biological Resources, Shanghai 200093, China.
- Shanghai Co-Innovation Center for Tumor Treatment with Energy, Shanghai 200093, China.
- Shanghai Origincell Biological Cryo Equipment Co., Ltd., Shanghai 200131, China.
*Corresponding authors’ E-mail: blliuk@163.com
Abstract
Background
Sperm cryopreservation is essential for assisted reproductive technologies, yet current methods face challenges regarding cryoprotectant toxicity and protocol standardization.
Objective
To develop an optimized cryopreservation system by investigating cryoprotective mechanisms and establishing standardized freezing protocols without using any animal-derived material.
Materials and methods
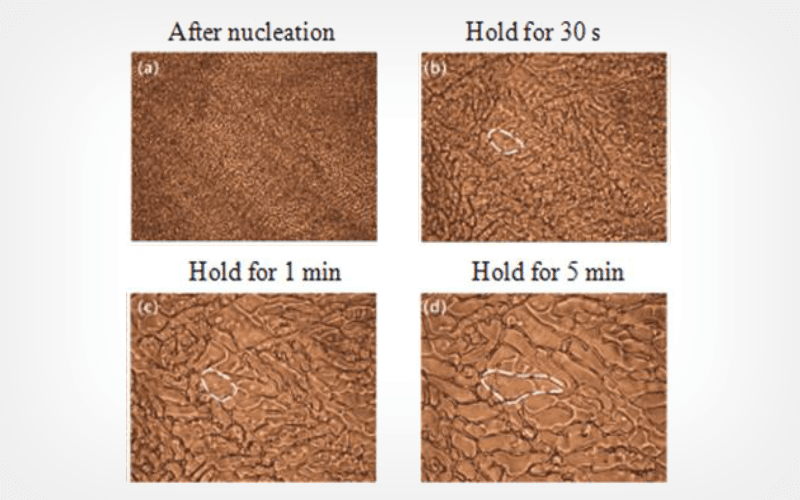
Thermodynamic analysis was performed to evaluate the cryoprotectant efficacy of glycerol (i.e., its ice crystal inhibition properties). An optimized formulation combining 10% glycerol with recombinant human serum albumin (rHSA) was developed to replace traditional egg yolk components. A novel freezing protocol incorporating gradient cooling and precise nucleation at -8.5°C was implemented using a custom-designed automated cryopreservation device (± 1°C precision).
Results
The optimized formulation significantly reduced the melting enthalpy while it maintained post-thaw sperm motility of 75.4 ± 3.2% and DNA fragmentation index <15%. The freezing protocol achieved 82.6 ± 4.1% recovery rate and reduced ice crystal formation by 62.3%. Preclinical study demonstrated significant improvements versus conventional methods (increased motility by 27.8% and enhanced DNA integrity by 34.6%, both p < 0.01).
Conclusion
The study established a standardized, pathogen-free cryopreservation system with demonstrated efficacy in preserving sperm quality. The findings provide both technical solutions for clinical practice and fundamental insights into cryoprotective mechanisms.
Keywords: induced nucleation; sperm cryopreservation.
CryoLetters 47 (2), 108-119 (2026)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr26210110712
Impact of vitrification cryopreservation on the transcriptomic profile of metaphase II mouse oocytes
Yuan Feng, Wenqing Yuan, Dan Zhao and Ming Cang*
- The State Key Laboratory of Reproductive Regulation and Breeding of Grassland Livestock, Inner Mongolia University, Hohhot, 010000, China.
*Corresponding authors’ E-mail: cangming@imu.edu.cn
Abstract
Background
Vitrification freezing has been shown to reduce mammalian oocyte developmental competence, and this has been strongly associated with abnormal mRNA expression in oocytes thawed by vitrification freezing. However the effect of vitrification freezing on the transcriptional machinery of oocytes examined by RNA sequencing has rarely been reported.
Objective
To study differentially expressed genes in mouse oocytes as a result of vitrification freezing.
Materials and methods
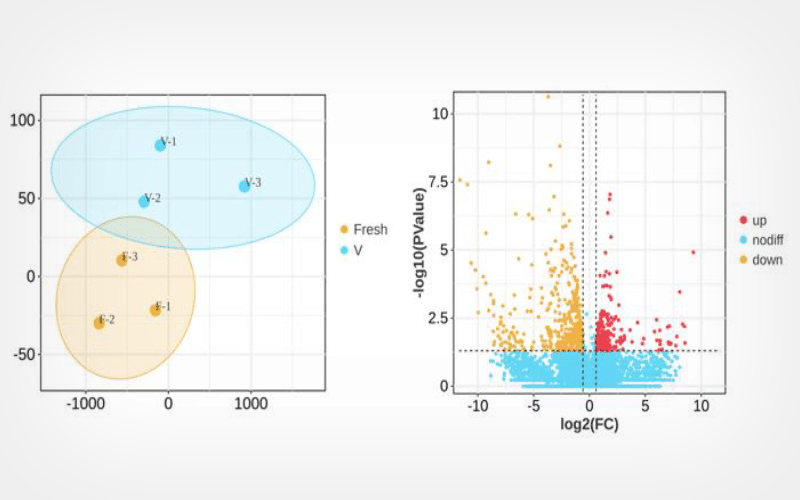
Differential gene expression was determined using DEseq2 (p-value <0.05, minimum multiplicity set at 1.5), and the transcriptomes of mouse stage MII oocytes from the Fresh and Vitrification groups were analyzed by employing the Smart-seq technique. The differentially expressed mRNAs were retrieved according to the Gene Ontology (GO) and KEGG databases, and finally, a real-time fluorescence quantitative PCR was used to characterize the reliability of the transcriptome data.
Results
Among them, 545 genes were detected to be up-regulated and 274 genes were up-regulated. Ten genes related to oxidative phosphorylation were screened to be down-regulated, and 44 genes were down-regulated with genes related to the HIF-signaling pathway, ribosomes and apoptosis.GO enrichment analysis showed that these genes were mainly concentrated in the cytoplasm, organelle membranes, mitochondrial membranes, and the Golgi apparatus. The four randomly selected candidate genes' mRNA expression levels were consistent with the results of RNA-seq.
Conclusion
Our results suggest that vitrification freezing affects mouse M II stage oocytes mainly through down-regulation of transcription, which leads to a decrease in the developmental capacity of vitrified oocytes. Our findings will help to identify ways to improve the vitrification efficiency of oocytes.
Keywords: apoptosis; cryopreservation; mitochondria; mouse oocytes; oxidative stress; vitrification.
CryoLetters 47 (2), 120-125 (2026)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr26210110212
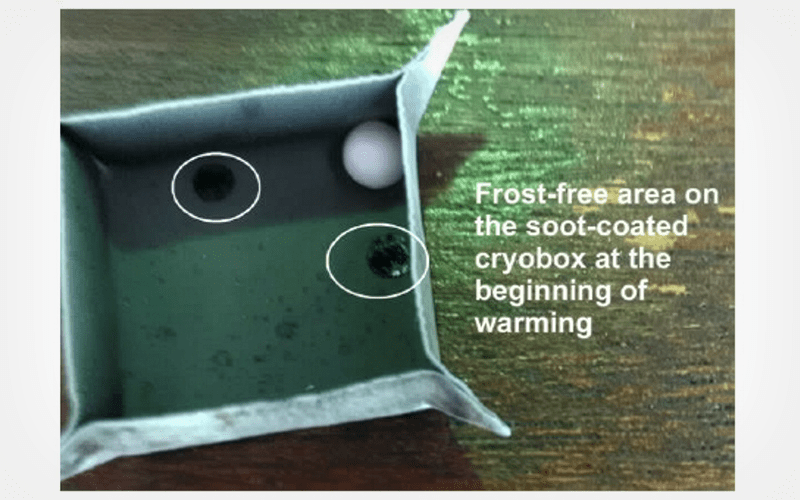
Permeable and non-permeable cryoprotectant-free cryopreservation of larger volumes native human semen via non-wettable rapeseed oil soot coatings
Karekin D. Esmeryan1* and Todor A. Chaushev2
- Acoustoelectronics Laboratory, Georgi Nadjakov Institute of Solid State Physics, Bulgarian Academy of Sciences, 72, Tzarigradsko Chaussee Blvd., 1784 Sofia, Bulgaria.
- Specialized Surgical Hospital “Doctor Malinov”, 46, Gotse Delchev blvd., 1860 Sofia, Bulgaria.
*Corresponding author’s E-mail: karekin_esmerian@abv.bg
Abstract
Background
The scientific advance in cryobiology allows the cryostorage of human semen without the use of toxic permeable cryoprotectants. To do this, the cellular suspensions are supplemented with non-permeable cryoprotective agents that possess mild cytotoxicity. Alternatively, the freezing is executed in vitrification solutions and preservation extenders, with preliminary selected spermatozoa via density-gradient centrifugation and swim-up techniques, although these procedures sometimes cause sperm DNA fragmentation.
Objective
To introduce unique results concerning the cryopreservation of larger volumes native human semen (above 20–30 µL) on water-repellent soot-coated surfaces, eliminating the involvement of any freezing extenders and formulations.
Results
The natural biomolecular matrix of seminal plasma combined with the icephobicity of rapeseed oil soot promoted the survival of over 5,500,000 forward-moving spermatozoa in 1 mL liquefied ejaculate.
Conclusion
The future improvement of this technology may facilitate the assisted human reproduction, helping to solve the demographic problem in Europe.
Keywords: cryoprotectant-free cryopreservation; human semen; instant freezing; soot.
CryoLetters 47 (2), 126-161 (2026)
© CryoLetters, editor@cryoletters.org
doi.org/10.54680/fr26210110412
Abstracts: Society for Low Temperature Biology, 61st Meeting
11-13 September 2025, Olsztyn, Poland






